•Lamellar Bone
–Collagen fibers arranged in parallel layers
–Normal adult bone
•Woven Bone (non-lamellar)
–Randomly oriented collagen fibers
–In adults, seen at sites of fracture healing, tendon or ligament attachment and in
pathological conditions
Lamellar Bone
•Cortical bone:
–Comprised of osteons (Haversian systems)
–Osteons communicate with medullary cavity by Volkmann’s canals
Haversian System
•Osteon with central haversian canal containing
–Cells
–Vessels
–Nerves
•Volkmann’s canal
–Connects osteons
Lamellar Bone
•Cancellous bone (trabecular or spongy bone)
–Bony struts (trabeculae) that are oriented in direction of the greatest stress
Woven Bone
•Coarse with random orientation
•Weaker than lamellar bone
•Normally remodeled to lamellar bone
Bone Composition
•Cells
–Osteocytes
–Osteoblasts
–Osteoclasts
•Extracellular Matrix
–Organic (35%)
•Collagen (type I) 90%
•Osteocalcin, osteonectin, proteoglycans, glycosaminoglycans, lipids (ground substance)
–Inorganic (65%)
•Primarily hydroxyapatite Ca5(PO4)3(OH)2
Osteoblasts
•Derived from mesenchymal stem cells
•Line the surface of the bone and produce osteoid
•Immediate precursor is fibroblast-like preosteoblasts
Osteocytes
•Osteoblasts surrounded by bone matrix
–trapped in lacunae
•Function poorly understood
–regulating bone metabolism in response to stress and strain
Osteocyte Network
•Osteocyte lacunae are connected by canaliculi
•Osteocytes are interconnected by long cell processes that project through the canaliculi
•Preosteoblasts also have connections via canaliculi with the osteocytes
•Network probably facilitates response of bone to mechanical and chemical factors
Osteoclasts
•Derived from hematopoietic stem cells (monocyte precursor cells)
•Multinucleated cells whose function is bone resorption
•Reside in bone resorption pits (Howship’s lacunae)
•Parathyroid hormone stimulates receptors on osteoblasts that activate osteoclastic bone
resorption
Components of Bone Formation
•Cortex
•Periosteum
•Bone marrow
•Soft tissue
Prerequisites for Bone Healing
•Adequate blood supply
•Adequate mechanical stability
Mechanisms of Bone Formation
•Cutting Cones
•Intramembranous Bone Formation
•Endochondral Bone Formation
Cutting Cones
•Primarily a mechanism to remodel bone
•Osteoclasts at the front of the cutting cone remove bone
•Trailing osteoblasts lay down new bone
Intramembranous (Periosteal) Bone Formation
•Mechanism by which a long bone grows in width
•Osteoblasts differentiate directly from preosteoblasts and lay down seams of osteoid
•Does NOT involve cartilage anlage
Endochondral Bone Formation
•Mechanism by which a long bone grows in length
•Osteoblasts line a cartilage precursor
•The chondrocytes hypertrophy, degenerate and calcify (area of low oxygen tension)
•Vascular invasion of the cartilage occurs followed by ossification (increasing oxygen
tension)
Blood Supply
•Long bones have three blood supplies
–Nutrient artery (intramedullary)
–Periosteal vessels
–Metaphyseal vessels
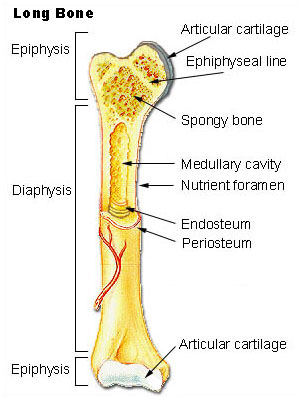
Nutrient Artery
•Normally the major blood supply for the diaphyseal cortex (80 to 85%)
•Enters the long bone via a nutrient foramen
•Forms medullary arteries up and down the bone
Periosteal Vessels
•Arise from the capillary-rich periosteum
•Supply outer 15 to 20% of cortex normally
•Capable of supplying a much greater proportion of the cortex in the event of injury to
the medullary blood supply
Metaphyseal Vessels
•Arise from periarticular vessels
•Penetrate the thin cortex in the metaphyseal region and anastomose with the medullary
blood supply
Vascular Response in Fracture Repair
•Fracture stimulates the release of growth factors that promote angiogenesis and
vasodilation
•Blood flow is increased substantially to the fracture site
–Peaks at two weeks after fracture
Mechanical Stability
•Early stability promotes revascularization
•After first month, loading and interfragmentary motion promotes greater callus
formation
•Mechanical load and small displacements at the fracture site stimulate healing
•Inadequate stabilization may result in excessive deformation at the fracture site
interrupting tissue differentiation to bone (soft callus)
•Over-stabilization, however, reduces periosteal bone formation (hard callus)
Stages of Fracture Healing
•Inflammation
•Repair
•Remodeling
Inflammation
•Tissue disruption results in hematoma at the fracture site
•Local vessels thrombose causing bony necrosis at the edges of the fracture
•Increased capillary permeability results in a local inflammatory milieu
–Osteoinductive growth factors stimulate the proliferation and differentiation of
mesenchymal stem cells
Repair
•Periosteal callus forms along the periphery of the fracture site
–Intramembranous ossification initiated by preosteoblasts
•Intramedullary callus forms in the center of the fracture site
–Endochondral ossification at the site of the fracture hematoma
•Chemical and mechanical factors stimulate callus formation and mineralization
Remodeling
•Woven bone is gradually converted to lamellar bone
•Medullary cavity is reconstituted
•Bone is restructured in response to stress and strain (Wolff’s Law)
Mechanisms for Bone Healing
•Direct (primary) bone healing
•Indirect (secondary) bone healing
Direct Bone Healing
•Mechanism of bone healing seen when there is no motion at the fracture site (i.e. rigid internal fixation)
•Does not involve formation of fracture callus
•Osteoblasts originate from endothelial and perivascular cells
•A cutting cone is formed that crosses the fracture site
•Osteoblasts lay down lamellar bone behind the osteoclasts forming a secondary osteon
•Gradually the fracture is healed by the formation of numerous secondary osteons
•A slow process – months to years
Components of Direct Bone Healing
•Contact Healing
–Direct contact between the fracture ends allows healing to be with lamellar bone
immediately
•Gap Healing
–Gaps less than 200-500 microns are primarily filled with woven bone that is
subsequently remodeled into lamellar bone
–Larger gaps are healed by indirect bone healing (partially filled with fibrous
tissue that undergoes secondary ossification)
Indirect Bone Healing
•Mechanism for healing in fractures that are not rigidly fixed.
•Bridging periosteal (soft) callus and medullary (hard) callus re-establish
structural continuity
•Callus subsequently undergoes endochondral ossification
•Process fairly rapid - weeks
Local Regulation of Bone Healing
•Growth factors
•Cytokines
•Prostaglandins/Leukotrienes
•Hormones
•Growth factor antagonists
Growth Factors
•Transforming growth factor
•Bone morphogenetic proteins
•Fibroblast growth factors
•Platelet-derived growth factors
•Insulin-like growth factors
Transforming Growth Factor
•Superfamily of growth factors (~34 members)
•Act on serine/threonine kinase cell wall receptors
•Promotes proliferation and differentiation of mesenchymal precursors for
osteoblasts, osteoclasts and chondrocytes
•Stimulates both endochondral and intramembranous bone formation
–Induces synthesis of cartilage-specific proteoglycans and type II collagen
–Stimulates collagen synthesis by osteoblasts
Bone Morphogenetic Proteins
•Osteoinductive proteins initially isolated from demineralized bone matrix
–Proven by bone formation in heterotopic muscle pouch
•Induce cell differentiation
–BMP-3 (osteogenin) is an extremely potent inducer of mesenchymal tissue
differentiation into bone
•Promote endochondral ossification
–BMP-2 and BMP-7 induce endochondral bone formation in segmental defects
•Regulate extracellular matrix production
–BMP-1 is an enzyme that cleaves the carboxy termini of procollagens I, II and
III
Bone Morphogenetic Proteins
•These are included in the TGF-β family
–Except BMP-1
•BMP2-7,9 are osteoinductive
•BMP2,6, & 9 may be the most potent in osteoblastic differentiation
•Work through the intracellular Smad pathway
•Follow a dose/response ratio
BMP Antagonists
•May have important role in bone formation
•Noggin
–Extra-cellular inhibitor
–Competes with BMP-2 for receptors
BMP Future Directions
•BMP-2
–Increased fusion rate in spinal fusion
•BMP-7 equally effective as ICBG in nonunions
•Must be applied locally because of rapid systemic clearance
•? Effectiveness in acute fractures
•? Increased wound healing in open injuries
•Protein therapy vs. gene therapy
Fibroblast Growth Factors
•Both acidic (FGF-1) and basic (FGF-2) forms
•Increase proliferation of chondrocytes and osteoblasts
•Enhance callus formation
•FGF-2 stimulates angiogenesis
Platelet-Derived Growth Factor
•A dimer of the products of two genes, PDGF-A and PDGF-B
–PDGF-BB and PDGF-AB are the predominant forms found in the circulation
•Stimulates bone cell growth
•Mitogen for cells of mesenchymal origin
•Increases type I collagen synthesis by increasing the number of osteoblasts
•PDGF-BB stimulates bone resorption by increasing the number of osteoclasts
Insulin-like Growth Factor
•Two types: IGF-I and IGF-II
–Synthesized by multiple tissues
–IGF-I production in the liver is stimulated by Growth Hormone
•Stimulates bone collagen and matrix synthesis
•Stimulates replication of osteoblasts
•Inhibits bone collagen degradation
Cytokines
•Interleukin-1,-4,-6,-11, macrophage and granulocyte/macrophage (GM) colony-
stimulating factors (CSFs) and Tumor Necrosis Factor
•Stimulate bone resorption
–IL-1 is the most potent
•IL-1 and IL-6 synthesis is decreased by estrogen
–May be mechanism for post-menopausal bone resorption
•Peak during 1st 24 hours then again during remodeling
•Regulate endochondral bone formation
Prostaglandins / Leukotrienes
•Effect on bone resorption is species dependent and their overall effects in humans
unknown
•Prostaglandins of the E series
–Stimulate osteoblastic bone formation
–Inhibit activity of isolated osteoclasts
•Leukotrienes
–Stimulate osteoblastic bone formation
–Enhance the capacity of isolated osteoclasts to form resorption pits
Hormones
•Estrogen
–Stimulates fracture healing through receptor mediated mechanism
–Modulates release of a specific inhibitor of IL-1
•Thyroid hormones
–Thyroxine and triiodothyronine stimulate osteoclastic bone resorption
•Glucocorticoids
–Inhibit calcium absorption from the gut causing increased PTH and therefore
increased osteoclastic bone resorption
•Parathyroid Hormone
–Intermittent exposure stimulates
•Osteoblasts
•Increased bone formation
•Growth Hormone
–Mediated through IGF-1 (Somatomedin-C)
–Increases callus formation and fracture strength
Vascular Factors
•Metalloproteinases
–Degrade cartilage and bones to allow invasion of vessels
•Angiogenic factors
–Vascular-endothelial growth factors
•Mediate neo-angiogenesis & endothelial-cell specific mitogens
–Angiopoietin (1&2)
•Regulate formation of larger vessels and branches
Local Anatomic Factors That Influence Fracture Healing
•Soft tissue injury
•Interruption of local blood supply
•Interposition of soft tissue at fracture site
•Bone death caused by radiation, thermal or chemical burns or infection
Systemic Factors That Decrease Fracture Healing
•Malnutrition
–Causes reduced activity and proliferation of osteochondral cells
–Decreased callus formation
•Smoking
–Cigarette smoke inhibits osteoblasts
–Nicotine causes vasoconstriction diminishing blood flow at fracture site
•Diabetes Mellitus
–Associated with collagen defects including decreased collagen content, defective cross-
linking and alterations in collagen sub-type ratios
Electromagnetic Field
•In vitro bone deformation produces piezoelectric currents and streaming potentials
•Electromagnetic (EM) devices are based on Wolff’s Law that bone responds to
mechanical stress: Exogenous EM fields may simulate mechanical loading and
stimulate bone growth and repair
•Clinical efficacy very controversial
Types of EM Devices
•Microamperes
•Direct electrical current
•Capacitively coupled electric fields
•Pulsed electromagnetic fields (PEMF)
PEMF
•Approved by the FDA for the treatment of non-unions
•Efficacy of bone stimulation appears to be frequency dependant
–Extremely low frequency (ELF) sinusoidal electric fields in the physiologic
range are most effective (15 to 30 Hz range)
–Specifically, PEMF signals in the 20 to 30 Hz range (postural muscle activity)
appear more effective than those below 10 Hz (walking)
Ultrasound
•Low-intensity ultrasound is approved by the FDA for stimulating healing of fresh
fractures
•Modulates signal transduction, increases gene expression, increases blood flow,
enhances bone remodeling and increases callus torsional strength in animal models
•Human clinical trials show a decreased time of healing in fresh fractures
•Has also been shown to decrease the healing time in smokers potentially reversing the ill
effects of smoking
Summary
•Fracture healing is influenced by many variables including mechanical stability,
electrical environment, biochemical factors and blood flow
•Our ability to enhance fracture healing will increase as we better understand the
interaction between these variables